GROUP LEADER: Prof. Erick Strauss
Link to own research page
Position: Professor
Office: A118 JC Smuts Building
Phone: +27-(0)21-808-5866
Fax: +27-(0)21-808-5863
Email: estrauss@sun.ac.za
Educational Background
PhD, Cornell University (USA), 2003
Awards
Raikes Medal, South African Chemical Institute (2013)
Beckman-Coulter Silver Medal, South African Society for Biochemistry and Molecular Biology (2010)
NRF President's Award (P-rating) (2008-2012)
Rector's Award for Outstanding Teaching, Stellenbosch University (2007)
Research Emphasis
Chemical Biology, Mechanistic Enzymology, Antimicrobial Drug Design & Discovery, Biocatalysis
Research Summary
The Strauss Lab's core research efforts are in the multidisciplinary field of Chemical Biology. We mainly focus on the chemistry and biology of the ubiquitous metabolic cofactor Coenzyme A, and on low molecular weight thiol-dependent redox biology. We also apply the expertise we gain in this manner to the design and development of new antimicrobial agents – especially those that target diseases relevant in the African health context, such as malaria and tuberculosis.
Research Description
Interests
Research in the Strauss group is broadly focused on increasing our understanding of the enzymology of coenzyme A (CoA) and other medicinally-relevant low molecular weight thiols, and applying this knowledge in biocatalysis and antibiotic drug development. Our goal is to identify new drug targets in important human pathogens such as Staphylococcus aureus, Mycobacterium tuberculosis and Plasmodium falciparum that exploit their dependence on these essential cofactors.
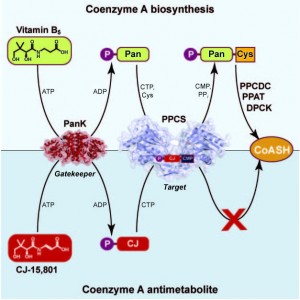
Our research strategy focuses on elucidating and studying all aspects of the enzymes involved in the biosynthetic pathway of the thiol-containing cofactor, and applying this knowledge in the design of inhibitors of these enzymes as well as those that use the cofactor. Often such inhibitors are also analogues of the native cofactor, and therefore can be prepared biocatalytically by co-opting the natural biosynthetic enzymes. In some cases this occurs naturally as shown in the figure below for the natural product CJ-15,801, which uses this as part of its inhibition strategy.
Tools
We use a broad range of tools and techniques in our laboratories, and you may expect to encounter people doing molecular biology, protein expression and purification, assay development, and also organic synthesis. Our labs are well-equipped to perform these studies, and we are heavy users of the university's excellent MS facility which offers LC/MS and MALDI services. We also work closely with the synthetic organic group of Prof. Willem van Otterlo in the Department of Chemistry and Polymer Science.
Collaborations
We collaborate extensively with several international research groups in the US, Spain, Italy and Australia. Collaborations that have been especially fruitful, are those with the lab of Hong Zhang at the University of Texas Southwestern Medical Center in the US on the structural biology of pantothenate kinases, with the group of Joaquín Ariño at the Universitat Autonoma de Barcelona in Barcelona, Spain on CoA biosynthesis in yeast and with Kevin Saliba's group at the Australian National University in Canberra, Australia on the development of antiplasmodial agents that target CoA biosynthesis and utilization.
Selected Publications
Full list available at ReseachGate
Variation in pantothenate kinase type determines the pantothenamide mode of action and impacts on coenzyme A salvage biosynthesis. M. de Villiers, L. Barnard, L. Koekemoer, J.L. Snoep & E. Strauss. FEBS J. 281, 4731–4753 (2014).
Recent advances in targeting coenzyme A biosynthesis and utilization for antimicrobial drug development. W.J,A. Moolman, M. de Villiers, & E. Strauss. Biochem. Soc. Trans. 42, 1080–1086 (2014).
Structural modification of pantothenamides counteracts degradation by pantetheinase and improves antiplasmodial activity. M. de Villiers, C.J. Macuamule, C. Spry, Y.-M. Hyun, E. Strauss and K.J. Saliba. ACS Med. Chem. Lett. 4, 784−789 (2013).
Pantothenamides are potent, on-target inhibitors of Plasmodium falciparum growth when serum pantetheinase is inactivated. C. Spry, C. Macuamule, Z. Lin, K.G. Virga, R.E. Lee, E. Strauss and K.J. Saliba. PLoS ONE, 8(2): e54974 (2013).
The Antibiotic CJ-15,801 is an Antimetabolite which Hijacks and then Inhibits CoA Biosynthesis. R. van der Westhuyzen, J.C. Hammons, J.L. Meier, S. Dahesh, W.J.A. Moolman, S.C. Pelly, V. Nizet, M.D. Burkart and E. Strauss. Chem. Biol. 19, 559–571 (2012).
Functional Mapping of the Disparate Activities of the Yeast Moonlighting Protein Hal3. J.A. Abrie, A. González, E. Strauss and J. Ariño. Biochem. J. 442, 357-368 (2012).
Grand challenge commentary: Exploiting single-cell variation for new antibiotics. E. Strauss. Nat. Chem. Biol. 6, 873–875, (2010).
Biocatalytic Production of Coenzyme A Analogues. E. Strauss, M. de Villiers and I. Rootman. ChemCatChem 2, 929-937, (2010).
Michael acceptor-containing coenzyme A analogues as inhibitors of the atypical coenzyme A disulfide reductase fromStaphylococcus aureus. R. van der Westhuyzen and E. Strauss. J. Am. Chem. Soc. 132, 12853-12855 (2010).
Moonlighting proteins Hal3 and Vhs3 form a heteromeric PPCDC with Ykl088w in yeast CoA biosynthesis. A. Ruiz, A. González, I. Muñoz, R. Serrano, J.A. Abrie, E. Strauss, and J. Ariño. Nature Chem. Biol. 5, 920-928, (2009).

